Patients providing their experiences were compensated for their time. Other patients may have a different experience.
How does NERLYNX work?
NERLYNX is a small molecule targeted therapy that works inside your cells and helps stop HER2 protein signals.
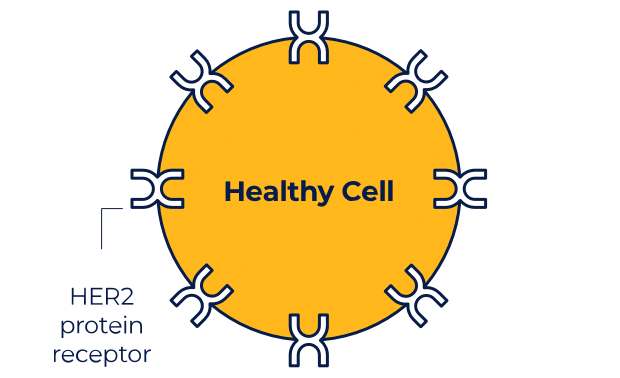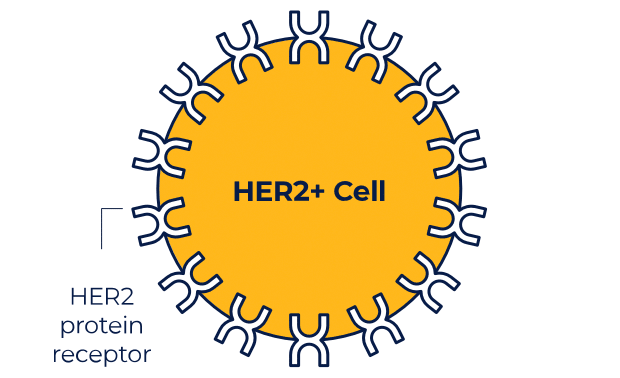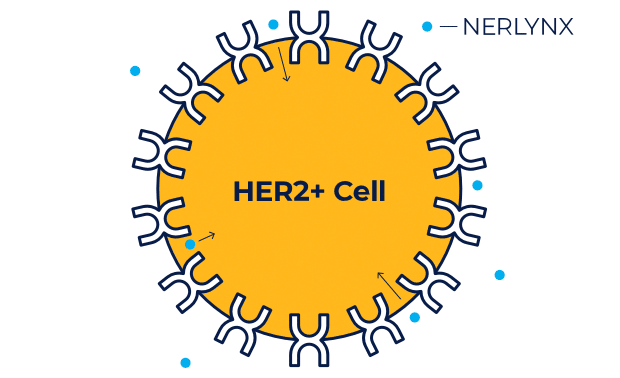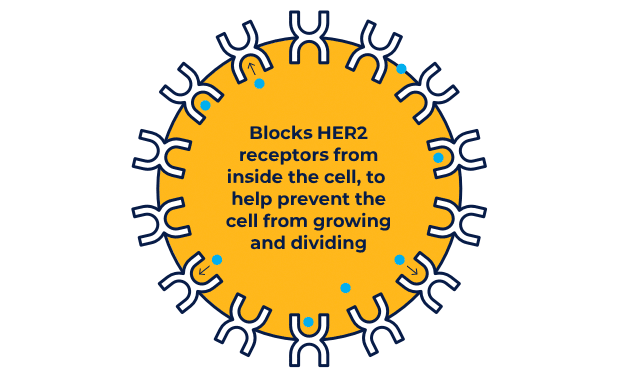
NERLYNX in women with early-stage and metastatic HER2+ breast cancer
In early-stage HER2+ breast cancer
What should I know about how NERLYNX was studied?
NERLYNX was studied in a phase 3 trial examining the safety and effectiveness of NERLYNX vs placebo. The clinical trial included 2,840 people who had early-stage HER2+ breast cancer.

In a clinical study, NERLYNX reduced the risk of recurrence by 34% at 2 years. This was the primary endpoint of the trial.
In absolute numbers, 94.2% of the 1,420 women who took NERLYNX in the study did not have cancer return after 2 years, compared to 91.9% of the 1,420 women who took placebo.
Individual results may vary.
NERLYNX was studied in people with early-stage HER2+ breast cancer who were HR+ as well. After the main portion of the trial, 75% of the women (2,117 out of 2,840) agreed to continue being evaluated. At 5 years from the start of the original trial, a special analysis looked at 1,334 of the women with HR+ breast cancer. Of these women, 670 started NERLYNX and 664 started placebo within 1 year of treatment with trastuzumab. Most of these women received endocrine therapy while on NERLYNX.

A reduction in the risk of recurrence was observed.
90.8% of the 670 women in the NERLYNX group did not have their cancer return after 5 years, compared to 85.7% of the 664 women who took placebo.
Individual results may vary.
Talk to your doctor for more information on NERLYNX data. Your doctor will help you manage expectations.
Is there more about the 5-year special analysis that I should know about?
This 5-year analysis also observed a 59% reduction in the risk of central nervous system (CNS) metastases among women with early-stage HER2+ HR+ breast cancer who started NERLYNX within 1 year of treatment with trastuzumab. A CNS metastasis is cancer that has spread to parts of the CNS, such as the brain or spinal cord.
In absolute numbers, 98.4% of the 670 women who took NERLYNX did not experience a CNS recurrence or death from any cause, compared to 95.7% of the 664 women who took placebo.
Individual results may vary.
With NERLYNX, you may help further reduce the risk that your HER2+ breast cancer will return.
Mentor program*
If you are considering or already being treated with NERLYNX, our mentor program connects you with someone who has taken NERLYNX.†
We’re ready to chat!
To talk to a mentor, call 1‑855‑816‑5421.
*The mentor program is here for you with support and resources. Your healthcare team should always be your primary source of information for treatment decisions.
†Puma mentors are compensated for their time.
I have early-stage HER2+ breast cancer. How do I take my NERLYNX?
NERLYNX is an oral medicine that you take once a day at home. Talk to your doctor about the correct dosage for you. Your doctor may start you off with a lower dose of NERLYNX and then gradually increase to your full dosage.
Here’s an example of what dose escalation with NERLYNX may look like:


Your doctor may also prescribe you an antidiarrheal such as loperamide when you start treatment and then as needed. Talk to your doctor about how much and how often to take this medicine. Take a look at our Antidiarrheal Support Program to see if it could help you.‡


‡Limitations apply. Puma Biotechnology reserves the right to rescind, revoke, or amend this program without notice. For full terms and conditions, call 1-855-816-5421.
§Take NERLYNX exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dosage of NERLYNX if needed.
For more information on the benefits of NERLYNX, download the patient brochure.
What side effects could I experience?
Common side effects
Diarrhea is the most common side effect of taking NERLYNX, and can be severe. Diarrhea may lead to loss of too much body salt and fluid, which can cause dehydration.
Talk to your doctor about how to best manage NERLYNX side effects, and call them right away if you have severe diarrhea or experience diarrhea along with weakness, dizziness, or fever.
Here are some other commonly reported side effects when NERLYNX is used alone to treat early-stage HER2+ breast cancer:
- Nausea
- Stomach-area (abdomen) pain
- Tiredness
- Vomiting
- Rash
- Dry or inflamed mouth, or mouth sores
- Decreased appetite
- Muscle spasms
- Upset stomach
- Nail problems including color change
- Dry skin
- Swelling of your stomach-area
- Nosebleed
- Weight loss
- Urinary tract infection
These are not all of the possible side effects of NERLYNX. Tell your healthcare provider if you have any side effects that bother you or that does not go away. You may report side effects to FDA at 1-800-FDA-1088.
Please see Important Safety Information below.
With metastatic HER2+ breast cancer
Take a stand against progression
“Metastatic” means that the cancer has spread beyond the breast and nearby lymph nodes to other parts of the body. NERLYNX plus capecitabine (an oral chemotherapy) can be taken to help prevent advanced or metastatic HER2+ breast cancer from spreading further.
Who is NERLYNX right for?
If you've already been treated with 2 or more HER2-directed therapies for metastatic breast cancer (like trastuzumab, T-DM1, and/or pertuzumab), NERLYNX plus capecitabine may be right for you.
What should I know about the benefits of NERLYNX plus capecitabine?
NERLYNX was studied in a phase 3 trial examining the safety and effectiveness of NERLYNX in combination with capecitabine vs lapatinib plus capecitabine. The clinical trial included 621 people with metastatic HER2+ breast cancer who had previously been treated with 2 or more HER2-directed therapies.

In a clinical study, NERLYNX plus capecitabine reduced the risk of progression by 24%.
These are relative benefits. In absolute numbers, 29% of the 307 people in the NERLYNX + capecitabine group did not see their cancer progress over 1 year compared to 15% of the 314 people in the lapatinib + capecitabine group.
Individual results may vary.
Talk to your doctor for more information on NERLYNX data. Your doctor will help you manage expectations.

In the clinical trial, NERLYNX also gave people a longer response to treatment compared with lapatinib plus capecitabine.*
*Median of 8.5 months compared to 5.6 months for those taking lapatinib. The 8.5- and 5.6-months durations were calculated only from the percentage of people that responded to therapy (32.8% in the NERLYNX group vs 26.7% in the lapatinib group).
Mentor program†
If you are considering or already being treated with NERLYNX, our mentor program connects you with someone who has taken NERLYNX.‡
We're ready to chat!
To talk to a mentor, call 1‑855‑816‑5421.
†The mentor program is here for you with support and resources. Your healthcare team should always be your primary source of information for treatment decisions.
‡Puma mentors are compensated for their time.
I have metastatic breast cancer. How do I take my NERLYNX?
NERLYNX is an oral medicine that you take once a day. Talk to your doctor about the correct dosage for you. Your doctor may start you off with a lower dose of NERLYNX and then gradually increase to your full dosage.
It's important to remember if you're taking NERLYNX for your metastatic breast cancer, you must take it in combination with capecitabine.
Here’s an example of what dose escalation with NERLYNX may look like:




Your doctor may also prescribe you an antidiarrheal such as loperamide when you start treatment and then as needed. Talk to your doctor about how much and how often to take this medicine. Take a look at our Antidiarrheal Support Program to see if it could help you.§
§Limitations apply. Puma Biotechnology reserves the right to rescind, revoke, or amend this program without notice. For full terms and conditions, call 1-855-816-5421.
For more information on the benefits of NERLYNX, download the patient brochure.
What side effects could I experience?
Common side effects for NERLYNX plus capecitabine
Diarrhea is the most common side effect of taking NERLYNX, and can be severe. Diarrhea may lead to loss of too much body salt and fluid, which can cause dehydration.
Talk to your doctor about how to best manage NERLYNX side effects, and call them right away if you have severe diarrhea or experience diarrhea along with weakness, dizziness, or fever.
Here are some other commonly reported side effects when NERLYNX is used in combination with capecitabine to treat metastatic HER2+ breast cancer:
- Nausea
- Vomiting
- Decreased appetite
- Constipation
- Tiredness/weakness
- Weight loss
- Dizziness
- Back pain
- Joint pain
- Urinary tract infection
- Upper respiratory tract infection
- Swelling of your stomach-area
- Kidney problems
- Muscle spasms
These are not all of the possible side effects of NERLYNX. Tell your healthcare provider if you have any side effects that bother you or that does not go away. You may report side effects to FDA at 1-800-FDA-1088.
Please see Important Safety Information below.
Tips for taking NERLYNX
Here are some tips and procedures for taking your NERLYNX from women and their caregivers who’ve been there:
Did you know there are educational programs and services that help make NERLYNX more accessible?
Check out Puma Patient Lynx™How do I manage side effects?
If side effects arise, talk to your healthcare provider.
Diarrhea is a common side effect of NERLYNX, but it can also be severe. Diarrhea may lead to loss of too much body salts and fluid, which can cause dehydration.
For patients with early-stage HER2+ breast cancer, your doctor may start you with a lower dose and slowly increase the dose until you are taking the recommended dosage of 6 pills a day. This can help you adjust to NERLYNX by managing this side effect.
Working with your healthcare team
Side effects can occur with NERLYNX. But there are steps you can take to help manage the side effect of diarrhea and see your treatment through.
Working with your healthcare team from the beginning is the key to success. Here are some simple steps to help prepare you:



Antidiarrheal Support Program∥
- With a prescription, patients can receive up to a 3-month supply of certain products used for antidiarrheal treatment for free
- Ask your healthcare team or a Puma network Specialty Pharmacy for a voucher
Puma offers vouchers that include access to a free supply of certain antidiarrheal medicines.
∥Limitations apply. Puma Biotechnology reserves the right to rescind, revoke, or amend this program without notice. For full terms and conditions, call 1-855-816-5421.
Our support services are designed with you in mind
Currently taking or thinking about taking NERLYNX? Sign up here to receive information and updates.



